Investigating effect of water of hydration on active pharmaceutical ingredients in a water-sensitive dosage form
Background
Preparation of mini buccal tablets incorporating either of the Alzheimer’s drugs, rivastigmine tartrate or donepezil hydrochloride, were developed for patients who have difficulty swallowing as a source of effervescence, by pairing the less commonly used malic acid with sodium bicarbonate in an experimentally determined (1:2) stochiometirc ratio.
Methods
To avoid premature reaction of the acid and the base during compounding, the tablet ingredients were mixed in the following order: acid, sweetener, binder, drug, preservative, base, and anti-adherent/lubricant.
Results
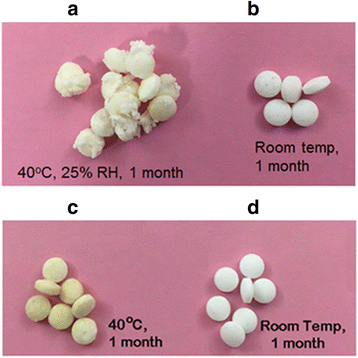
An accelerated thermal stability study at 40 °C and 25% relative humidity showed that the integrity of the effervescent tablets containing rivastigmine tartrate were superior to that of donepezil HCl tablets. FT-IR spectrometry confirmed the presence of water of hydrate in donepezil HCl crystals. This water was absent in the IR after one-month storage at accelerated thermal stability, but was present at room temperature. This behavior was not observed in the tablet made from rivastigmine tartrate powder. Differential scanning calorimetry of donepezil hydrochloride showed two thermal events: the first was associated with the loss of the water, and the second, at much higher temperature, was the melting of theanhydrous drug. Such behavior was not observed in the rivastigmine tartrate powder.
Conclusions
Hidden water which may function as catalyst to induce premature effervescence during storage.