Functionalized calcium carbonate (FCC) as a novel carrier to solidify supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS)
Abstract
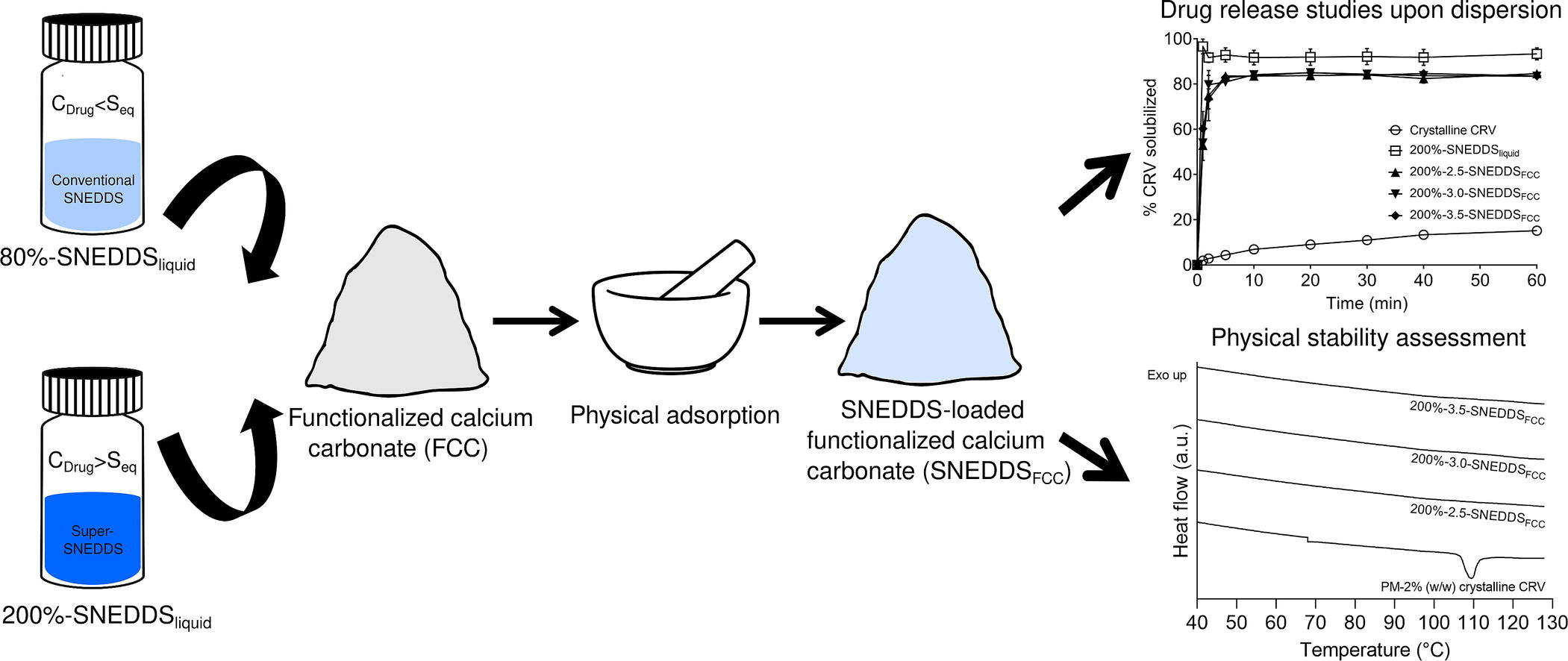
Functionalized calcium carbonate (FCC), a novel pharmaceutical excipient, has shown promising properties in the field of oral drug delivery. The current study aimed at evaluating the feasibility of FCC as a carrier for the solidification of self-nanoemulsifying drug delivery systems (SNEDDS) containing the poorly water-soluble model drug carvedilol (CRV). Conventional, subsaturated SNEDDS (80%-SNEDDSliquid) and supersaturated SNEDDS (200%-SNEDDSliquid) were loaded onto FCC via physical adsorption at three ratios; 2.5:1, 3.0:1 and 3.5:1 (w/w) of FCC:SNEDDSliquid, respectively, generating free-flowing powders (SNEDDSFCC) with drug loading ranging from 0.8% to 2.6% (w/w) CRV. The emulsification of SNEDDSFCC in a USP II dissolution setup (in purified water) was characterized using dynamic light scattering, resulting in similar droplet sizes and PDIs as observed for their liquid counterparts.
The morphology and physical state of the obtained SNEDDSFCC were characterized using scanning electron microscopy and differential scanning calorimetry. The physical stability and drug release upon dispersion were assessed as a function of storage time. The 200%-SNEDDSliquid were physically stable for 6 days, however, solidification using FCC stabilized the supersaturated concentrations of CRV for a test period of up to 10 weeks (solidification ratios 3.0:1 and 3.5:1 (FCC:SNEDDSliquid)). SNEDDSFCC achieved an improved rate and extent of drug release upon dispersion compared to the crystalline CRV in tap water (pH 7.5), however, to a lesser extent than their liquid counterparts. After 8 weeks of storage (25 °C at dry conditions), FCC was still able to rapidly release the SNEDDSliquid and demonstrated the same rate and extent of drug release as freshly prepared samples. The solidification of 200%-SNEDDSliquid in presence of FCC greatly improved the drug loading and showed an enhanced drug release profile compared to the conventional systems. In conclusion, FCC showed potential as a carrier for solidification of SNEDDS and for the development of novel supersaturated solid SNEDDS for the oral delivery of poorly water-soluble drugs.
Introduction
Oral drug delivery is the most favored route of drug administration due to its potential advantages including patient compliance, cost-effectiveness and non-invasiveness [1]. In a recent report (2021), 90% of the new drug candidates emerging from the pharmaceutical industry were classified as poorly water-soluble [2], leading to low and erratic bioavailability from conventional dosage forms after oral administration [3]. Hence, enabling formulation strategies are needed to improve the bioavailability of poorly water-soluble drugs after oral administration [4].
Lipid-based formulations (LBF) offer a solution by mitigating the inherently slow dissolution process of poorly water-soluble drugs due to their ability to pre-dissolve lipophilic drugs and thus circumvent the dissolution step in the gastrointestinal tract [5], [6], [7]. Amongst the various LBF, the use of a self-nanoemulsifying drug delivery system (SNEDDS) has attracted significant attention [8]. SNEDDS consists of drugs dissolved in an isotropic mixture of lipids, surfactants, co-surfactants and co-solvents from which a nanoemulsion is formed upon dispersion in aqueous media [7]. Conventional SNEDDS usually have a drug load between 50% to 90% of the drug’s equilibrium solubility (Seq) in the SNEDDS preconcentrate to avoid drug precipitation during storage and after administration [9]. The low drug loading of conventional SNEDDS potentially results in the administration of several dosage units, negatively affecting patient compliance [10]. To increase the drug loading in SNEDDS preconcentrate, Thomas et al. [9], [11] developed the concept of supersaturated SNEDDS (super-SNEDDS). Super-SNEDDS are characterized by containing drug concentrations well beyond the drug’s Seq in the SNEDDS preconcentrate and have been demonstrated to be suitable alternatives to conventional SNEDDS [9], [11], [12].
Despite the mentioned advantages, some limitations still exist for SNEDDS. They are usually filled in soft gelatin capsules, therefore limiting the drug loading by the fill weight and the drug solubility in the formulation [13], [14]. Furthermore, nearly all development and manufacturing activities involving soft gelatin capsules are outsourced to contract manufacturing organizations since most pharmaceutical companies lack in house capability leading to high production costs [15]. In an attempt to overcome these limitations, the solidification of liquid SNEDDS using chemically inert solid carriers to enable the production of solid dosage forms has attracted substantial interest in recent years [16].
The general hypothesis applied is that the development of solidified SNEDDS could combine the advantages of liquid SNEDDS with the high physical stability of solid dosage forms while enhancing or retaining the biopharmaceutical performance of the poorly water-soluble drugs compared to their liquid counterparts [17]. Moreover, the ease of manufacturing associated with low costs and the possibility of producing a wider range of dosage forms, e.g., powder filled in sachets or capsules or compressed into tablets, makes solidification attractive from an industrial perspective [16], [18], [19].
A plethora of research is devoted to the transformation of liquid LBF into solid dosage forms with a focus on various solidification methods as well as on the selection of solid carrier excipients. The solidification methods most commonly employed are physical adsorption, spray drying and melt extrusion [18]. A wide range of inorganic porous carriers for the solidification of LBF has been investigated including magnesium aluminometasilicate (e.g. Neusilin®), colloidal silicon dioxide (e.g. Aerosil®), porous amorphous silica gels (e.g. Sylysia® and Syloid®), and calcium silicate (e.g. Hubersorb®) [20]. An appropriate selection of the solid carrier for the solidification of LBF should enable the highest possible lipid (and drug) loading efficiency, adequate flowability, compactability, as well as an efficient re-dispersibility after administration [21], [22].
In order for the loaded poorly water-soluble drug to be released from the solid carrier, the liquid lipid phase of the solid dosage form has to desorb from the solid carrier and partition into the aqueous phase, or the solid carrier itself has to dissolve within the gastrointestinal tract [16]. Over the past years, various solid carriers have been investigated with respect to their role in retaining or enhancing the biopharmaceutical performance of solidified LBF. However, conflicting evidence has been reported as to whether the solidified formulations will preserve, enhance, or decrease the biopharmaceutical performance compared to their respective liquid counterparts. While several studies reported complete drug release from the solidified LBF [23], [24], [25], [26], others observed incomplete desorption from solidified formulations translating to a reduced in vivo performance [14], [27], [28], [29].
Neusilin®, a mesoporous magnesium aluminometasilicate, demonstrated significant potential as a solid carrier for LBF due to its ability to generate solidified LBF by simple physical adsorption and direct compactability allowing the manufacturing of tablets [30]. However, the suitability of Neusilin® as a solid carrier for LBF has been discussed controversially. Incomplete drug release was identified to be a major issue with formulations containing Neusilin® as a solid carrier due to the formation of gels by lipid-surfactants mixtures once in contact with water, hindering the drug release from deeper pores of Neusilin® [14], [15], [30]. A further reduction of drug release from the solidified LBF upon storage has been a concern, possibly due to the progressing migration of the mobile fractions of liquid LBF to deeper unwetted regions of the carrier during storage, entrapping the drug deeper within the pores [20].
The above-mentioned limitations drive the need to investigate the potential of new solid carriers for the solidification of LBF. In a previous study, solid supersaturatable SNEDDS loaded with glipizide were developed using conventional calcium carbonate, in combination with talc and hydroxypropyl methylcellulose (HPMC-E5) as polymeric precipitation inhibitor (PPI). However, the primary objective of the study was to evaluate the potential of HPMC-E5 in stabilizing the resulting supersaturated drug concentrations following dispersion [31].
Functionalized calcium carbonate (FCC) was recently identified as a novel pharmaceutical excipient for the oral delivery of poorly water-soluble drugs [32]. FCC is a microparticulate material ranging from 5-15 µm in diameter [33]. The small pore diameter (0.01-1 µm) and thus resulting high specific area of FCC enables water absorption at a faster rate and 10 times higher extent than conventional calcium carbonate [32], [34]. The physical attributes of FCC led to the exploration of this material in the field of pharmaceutical excipient research, resulting in the development of several innovative drug delivery systems. FCC has been investigated as mucoadhesive delivery systems for colon targeting [35], oral protein delivery systems [36], orally dispersible tablets [34], [37], floating tablets [38] and as a carrier for increasing the physical stability of amorphous drugs [39].
Since FCC has not been studied as a pharmaceutical excipient for the solidification of SNEDDS, the overall objective of this study was to investigate the feasibility of FCC to serve as a solid carrier for conventional SNEDDS and super-SNEDDS. It was hypothesized that the adsorption of super-SNEDDS onto FCC would increase the physical stability of the utilized BSC II model drug carvedilol (CRV) and help to achieve a greater CRV load. The developed solid (super-)SNEDDS and their liquid counterparts were characterized with respect to their in vitro performance including droplet size measurements and drug release upon dispersion. The developed solid (super-)SNEDDS were further characterized for drug release upon dispersion as a function of storage time.
Materials
Functionalized calcium carbonate (FCC) (Omyapharm® 500 – OG) was obtained from Omya International AG (Oftringen, Switzerland). Carvedilol (CRV) was purchased from Cipla Ltd. (Mumbai, India). Capmul MCM C8 EP/NF (medium chain (MC) mixed glycerides) and Captex 300 EP/NF (MC triglycerides) from Abitec (Columbus, OH, USA) were provided by Barentz (Odense, Denmark). Kolliphor RH40 (polyoxyl 40 hydrogenated castor oil) was donated by BASF (Ludwigshafen, Germany). Transcutol was donated by Gattefossé (Saint Priest, France). Potassium phosphate monobasic and potassium chloride were purchased from Sigma Aldrich (St Louis, MO, USA). Hydrochloric acid 37% (HCl) and acetonitrile (HPLC grade) were purchased from VWR Chemicals (Herlev, Denmark). Purified water was obtained from a SG Ultraclear water system (SG Water GmbH, Barsbüttel, Germany). Tap water (pH 7.5) was used through the course of the study.
Download the full study Journal Pre-proof as PDF here: Functionalized calcium carbonate (FCC) as a novel carrier to solidify supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS)
or read it here
Jumana Merchant, Anette Müllertz, Thomas Rades, Jacob Bannow, Functionalized calcium carbonate (FCC) as a novel carrier to solidify supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS), European Journal of Pharmaceutics and Biopharmaceutics, 2023, ISSN 0939-6411,
https://doi.org/10.1016/j.ejpb.2023.11.001.

